Cybin Inc. (NEO: CYBN) (NYSE American: CYBN), a biotechnology company focused on progressing psychedelic therapeutics, recently announced that the U.S. Food and Drug Administration (“FDA”) has authorized an Investigational New Drug (“IND”) application to proceed with the Company’s sponsored feasibility study using Kernel’s Flow technology to measure ketamine’s psychedelic effect on cerebral cortex hemodynamics.
“The word psychedelic means ‘mind-manifesting,’ but what has been missing is useful’ mind-imaging’—the ability to trace the neural correlates of human conscious experience dynamically. Conventional neuroimaging isn’t dynamic enough to study the psychedelic experience in the brain as it happens. This study of ketamine’s psychedelic effects while wearing headgear equipped with sensors to record brain activity could open up new frontiers of understanding,” said Dr. Alex Belser, Cybin’s Chief Clinical Officer.
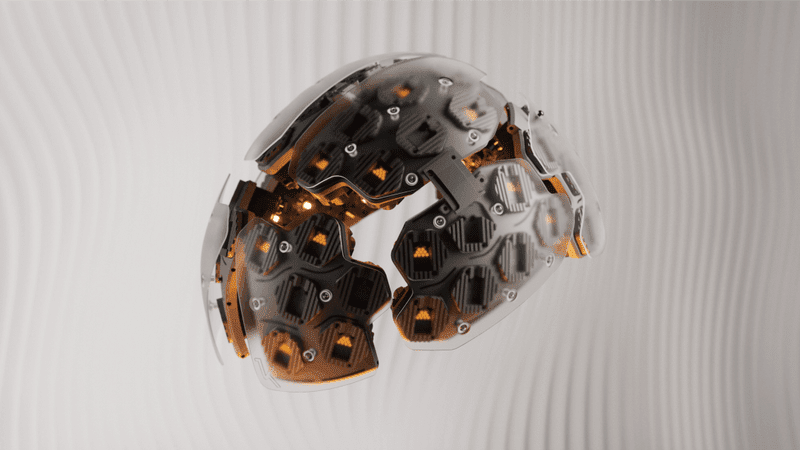
FDA Approves Investigational New Drug Authorization of Cybin’s Sponsored Feasibility Study using Kernel Flow.
Leveraging Kernel’s quantitative neuroimaging technology (“Kernel Flow”) may lead to new frontiers in psychedelic therapeutics by enabling the acquisition of longitudinal brain activity before, during, and after a psychedelic experience, providing quantification of what was previously subjective patient reporting.

“Quantitatively measuring the brain within the context of a psychedelic experience is a promising frontier,” said Bryan Johnson, founder and Chief Executive Officer of Kernel. “With Kernel Flow, Cybin’s researchers can start putting numbers and quantification to subjective states of mind, including altered ones.”
Kernel Flow uses pulsed light instead of continuous-wave light to increase measured brain information. In contrast with electroencephalography (“EEG”) electrodes that usually require gel on the head or functional magnetic resonance imaging (“fMRI”) studies that require a participant to lie in a scanner, Kernel Flow is effortlessly wearable. The entire system is the size and look of a bicycle helmet and could, in the future, to use more broadly for neuroscientific or physiological studies of brain activity during psychedelic use.
As part of Cybin’s sponsorship of the feasibility study, the Company will retain exclusive interest in any innovations discovered or developed through its independent analysis of the study findings. Kernel will hold the same rights relating to its Kernel technology.
“We hope this feasibility study can bridge the gap of real-time quantitative data collection during psychedelic treatments to further understand the correlation of effects from these powerful molecules. The ability to access real-time brain activity data during a psychedelic experience has tremendous potential for the development of future psychedelic therapeutics,” stated Doug Drysdale, Chief Executive Officer of Cybin.
In the past, the onus was on the patient to self-report subjective feedback to their healthcare provider. With Kernel, it may be easier to determine a patient’s specific reaction to treatment.

Leadership Q & A at the Wonderland conference
Cybin’s leadership, including Drysdale and the Company’s senior scientific research team, are hosting a Research & Development (R&D) day in conjunction with the Wonderland conference, on November 8, in Miami, Florida.
The in-person session will take place at the Adrienne Arsht Center for the Performing Arts, within the Green Room. Log-in details for the webcast for virtual attendees will be released a few days prior to the event. The event will be followed by a Q & A session.
The Company’s comparative pre-clinical data on CYB003 demonstrates multiple potential advantages over classical psychedelic molecules that include faster onset of action, shorter duration of action, excellent oral bioavailability, significant brain penetration, and significantly less inter-subject variability.
Progress on the R & D Front
Cybin’s Research and Development team has completed 74 in-vitro and in-vivo evaluations of Cybin’s expanding portfolio of psychedelic compounds being designed for potential therapeutic applications for several mental health conditions. To date, more than 50 novel compounds have been evaluated through collaborations with experienced contract research organizations for pharmacokinetic/pharmacodynamic profile, metabolic stability, receptor binding, and safety in order to identify preferred candidates for further development.
“Cybin continues to demonstrate superior properties of its CYB003 and CYB004 programs as we progress toward first-in-human studies, expected in early 2022. These experiments greatly expand our understanding of the potential therapeutic value of the studied compounds and further demonstrate Cybin’s strong research and development capabilities,” said Doug Drysdale, Cybin’s CEO.
Cybin aims to create a world-class portfolio of psychedelic molecules that can become commercially viable drug candidates.